연구동향
2023년 미국 FDA 55종의 신약 허가승인
작성자
관리자
작성일
2024-01-22 16:16
조회
563
미국에서 의약품을 판매하기 위해서는 필수적으로 미국 FDA의 심사를 거쳐서 승인을 받아야 한다.
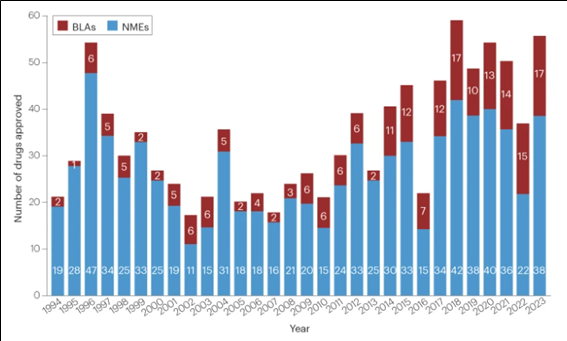
2023년 한해동안 미국 FDA의 의약품 평가연구센터(CDER: Center for Drug Evaluation and Research)는 총 55종의 신약을 승인하였다. 2022년 허가신약 37건 대비 약 50% 증가한 수치이다.
2023년 승인된 55건의 신약중 28건(51%)이 희귀의약품 지정(Orphan Drug Designation, ODD)을 통하여 승인을 받았으며, 20건(36%)이 혁신신약 (first-in-class)으로 분류되었다.
2023년 승인된 55건의 신약에 대하여
1. 적응증별로 보면,
2. 약물유형(Modality)별로 보면,
3. 신속심사 프로그램 활용
55종 신약별 상세한 적응증, 작용기전, 약물유형, 약물구조에 대한 정보는 아래 참조
2023년 1월~4월 사이에 승인된 14종의 신약(순번 1~14)에 대한 정보(바로가기)
2023년 5월~6월 사이에 승인된 12종의 신약(순번 15~26)에 대한 정보(바로가기)
2023년 7월~8월 사이에 승인된 9종의 신약(순번 27~35)에 대한 정보(바로가기)
2023년 9월~12월 사이에 승인된 20종의 신약(순번 36~55)에 대한 정보(바로가기)
2023년 한해동안 미국 FDA의 의약품 평가연구센터(CDER: Center for Drug Evaluation and Research)는 총 55종의 신약을 승인하였다. 2022년 허가신약 37건 대비 약 50% 증가한 수치이다.
2023년 승인된 55건의 신약중 28건(51%)이 희귀의약품 지정(Orphan Drug Designation, ODD)을 통하여 승인을 받았으며, 20건(36%)이 혁신신약 (first-in-class)으로 분류되었다.
<연도별 FDA 신약허가 건수: 1994~2023>
NMEs: New Molecular Entities(신규 저분자 합성신약), BLAs: Biologics License Applications(생물의약품 품목허가)
2023년 승인된 55건의 신약에 대하여
1. 적응증별로 보면,
항암제(Oncology ): 13건, 뇌 또는 신경관련 의약품(Neurology): 9건, 감염병 및 혈액관련 의약품(Infectious diseases & Haematology): 각 5건의 순서로 나타났다.
2. 약물유형(Modality)별로 보면,
저분자 합성신약: 34건,
단일클론항체신약 (monoclonal antibody): 12건,
단백질 의약품 (효소대체제, 호르몬, 융합단백질 등): 5건
올리고뉴클레오타이드 (siRNA, antisense ologonucleotides, RNA aptamer 등): 4건
3. 신속심사 프로그램 활용
또한 대부분 신약허가신청은 신속하게 심사를 받기 위하여 ‘신속심사프로그램’ 제도를 활용하여 진행되었다. (55건중 49건, 89%).
‘신속심사프로그램’을 이용한 허가심사유형별로 보면
-
- 패스트 트랙(Fast track): 25/55 (45%)
- 획기적 치료제 지정(Breakthrough Therapy Designation): 9/55 (16%)
- 우선 심사(Priority review): 31/55 (56%)
- 신속심사(Accelerated approval): 9/55 (16%)
<2023년 FDA 승인 55종 신약 목록>
| 순번 | 성분명
(제품명) |
개발사 | 작용기전(Properties) | 적응증(Indication) |
| 1 | Lecanemab (Leqembi) |
Eisai/Biogen | Amyloid-β-targeted mAb | Alzheimer disease |
| 2 | Bexagliflozin (Brenzavvy) | Theracosbio | SGLT2 inhibitor | Glycaemic control in type 2 diabetes mellitus |
| 3 | Pirtobrutinib (Jaypirca) |
Loxo/Eli Lilly | BTK inhibitor | Mantle cell lymphoma |
| 4 | Elacestrant (Orserdu) |
Stemline | ER antagonist | ER-positive, HER2-negative, ESR1-mutant breast cancer |
| 5 | Daprodustat (Jesduvroq) | GSK | HIF-PH inhibitor | Anaemia caused by CKD for adults on dialysis |
| 6 | Velmanase alfa (Lamzede) | Chiesi | Recombinant α-mannosidase | Non-CNS manifestations of α-mannosidosis |
| 7 | Sparsentan (Filspari) |
Travere | Endothelin and angiotensin II receptor antagonist | Proteinuria in primary IgA nephropathy |
| 8 | Omaveloxolone (Skyclarys) | Reata/Biogen | Mechanism unknown, NRF2 activator | Friedrich’s ataxia |
| 9 | Zavegepant (Zavzpret) |
Pfizer | CGRP receptor antagonist | Migraine |
| 10 | Trofinetide (Daybue) |
Acadia | Mechanism unknown | Rett syndrome |
| 11 | Retifanlimab (Zynyz) |
Incyte | PD1-targeted mAb | Merkel cell carcinoma |
| 12 | Rezafungin (Rezzayo) |
Cidara | Echinocandin antifungal | Candidemia and invasive candidiasis |
| 13 | Leniolisib (Joenja) |
Pharming | PI3Kδ inhibitor | Activated PI3Kδ syndrome |
| 14 | Tofersen (Qalsody) |
Biogen | SOD1-targeted ASO | SOD1 amyotrophic lateral sclerosis |
| 15 | Pegunigalsidase alfa (Elfabrio) | Chiesi | PEGylated recombinant α-galactosidase Α |
Fabry disease |
| 16 | Fezolinetant (Veozah) |
Astellas | Neurokinin 3 receptor antagonist | Hot flashes caused by menopause |
| 17 | Perfluorohexyloctane (Miebo) | Bausch + Lomb | Semifluorinated alkane | Dry eye disease |
| 18 | Epcoritamab (Epkinly) |
Genmab/AbbVie | CD20 × CD3 T-cell engager | DLBCL and high-grade B-cell lymphoma |
| 19 | Sulbactam/durlobactam (Xacduro) | Entasis | β-lactam antibacterial plus a β-lactamase inhibitor | Hospital-acquired and ventilator-associated bacterial pneumonia caused by susceptible ABC |
| 20 | Nirmatrelvir/ritonavir (Paxlovid) | Pfizer | SARS-CoV-2 main protease inhibitor plus a CYP3A inhibitor | Mild-to-moderate COVID-19 |
| 21 | Flotufolastat F18 (Posluma) | Blue Earth | Radioactive diagnostic agent | PET imaging in prostate cancer |
| 22 | Sotagliflozin (Inpefa) |
Lexicon | SGLT1/2 inhibitor | Heart failure |
| 23 | Glofitamab (Columvi) |
Genentech | CD20 × CD3 T-cell engager | DLBLC or large B-cell lymphoma |
| 24 | Ritlecitinib (Litfulo) |
Pfizer | JAK3 inhibitor | Alopecia areata |
| 25 | Rozanolixizumab (Rystiggo) | UCB | FcRn-targeted mAb | AChR- or MuSK-antibody-positive gMG |
| 26 | Somatrogon (Ngenla) |
Pfizer | Human growth hormone analogue | Growth hormone deficiency |
| 27 | Nirsevimab (Beyfortus) |
AstraZeneca | RSV F protein-targeted mAb | RSV lower respiratory tract disease |
| 28 | Quizartinib (Vanflyta) |
Daiichi Sankyo | FLT3 kinase inhibitor | AML |
| 29 | Lotilaner (Xdemvy) |
Tarsus | Ectoparasiticide | Demodex blepharitis |
| 30 | Zuranolone (Zurzuvae) |
Sage | GABAAreceptor PAM | Postpartum depression |
| 31 | Avacincaptad pegol (Izervay) | Iveric/Astellas | C5-targeted aptamer | Geographic atrophy secondary to AMD |
| 32 | Talquetamab (Talvey) |
Janssen | GPRC5D × CD3 T-cell engager | Multiple myeloma |
| 33 | Elranatamab (Elrexfio) |
Pfizer | BCMA × CD3 T-cell engager | Multiple myeloma |
| 34 | Palovarotene (Sohonos) | Ipsen | Retinoic acid receptor agonist | Fibrodysplasia ossificans progressiva |
| 35 | Pozelimab (Veopoz) |
Regeneron | C5-targeted mAb | CHAPLE disease |
| 36 | Motixafortide (Aphexda) | Biolinerx | CXCR4 inhibitor | Hematopoietic stem cell mobilization for autologous transplantation in multiple myeloma |
| 37 | Momelotinib (Ojjaara) |
GSK | JAK1/2, ALK2 inhibitor | Myelofibrosis in adults with anaemia |
| 38 | Gepirone (Exxua) |
Fabre-Kramer | 5HT1Areceptor agonist | Major depressive disorder |
| 39 | Cipaglucosidase alfa (Pombiliti) | Amicus | Recombinant α-glucosidase | Pompe disease |
| 40 | Nedosiran (Rivfloza) |
Novo Nordisk | LDHA-targeted siRNA | Primary hyperoxaluria type 1 |
| 41 | Etrasimod (Velsipity) |
Pfizer | S1P receptor modulator | Ulcerative colitis |
| 42 | Zilucoplan (Zilbrysq) |
UCB | Complement C5 inhibitor | AChR-antibody positive gMG |
| 43 | Bimekizumab (Bimzelx) |
UCB | IL-17A/F-targeted mAb | Plaque psoriasis |
| 44 | Vamorolone (Agamree) |
Santhera | Corticosteroid | Duchenne muscular dystrophy |
| 45 | Mirikizumab (Omvoh) |
Eli Lilly | IL-23-targeted mAb | Ulcerative colitis |
| 46 | Toripalimab (Loqtorzi) |
Coherus | PD1-targeted mAb | Nasopharyngeal carcinoma |
| 47 | Fruquintinib (Fruzaqla) |
Takeda | VEGFR1/2/3 kinase inhibitor | Colorectal cancer |
| 48 | Taurolidine/heparin (Defencath) | Cormedix | Thiadiazinane antimicrobial plus an anticoagulant | Incidence of catheter-related bloodstream infections |
| 49 | Repotrectinib (Augtyro) | Bristol Myers Squibb | ROS1 and TRK kinase inhibitor | ROS1-positive NSCLC |
| 50 | Efbemalenograstim alfa (Ryzneuta) | Evive | Recombinant leukocyte growth factor | Neutropenia |
| 51 | Capivasertib (Truqap) |
AstraZeneca | AKT kinase inhibitor | Breast cancer |
| 52 | Nirogacestat (Ogsiveo) |
Springworks | γ-secretase inhibitor | Desmoid tumours |
| 53 | Iptacopan (Fabhalta) |
Novartis | Complement factor B inhibitor | Paroxysmal nocturnal haemoglobinuria |
| 54 | Birch triterpenes (Filsuvez) | Chiesi | Mechanism unknown | Epidermolysis bullosa |
| 55 | Eplontersen (Wainua) |
Ionis/AstraZeneca | TTR-targeted ASO | hATTR with polyneuropathy |
55종 신약별 상세한 적응증, 작용기전, 약물유형, 약물구조에 대한 정보는 아래 참조
2023년 1월~4월 사이에 승인된 14종의 신약(순번 1~14)에 대한 정보(바로가기)
2023년 5월~6월 사이에 승인된 12종의 신약(순번 15~26)에 대한 정보(바로가기)
2023년 7월~8월 사이에 승인된 9종의 신약(순번 27~35)에 대한 정보(바로가기)
2023년 9월~12월 사이에 승인된 20종의 신약(순번 36~55)에 대한 정보(바로가기)
작성: 이현규 (한국화학연구원)
<참고자료>