연구동향
New Drug Candidates at AACR New Orleans 2022
Author
관리자
Date
2022-04-12 18:30
Views
4529
2022. 4.8-13. New Orleans에서 개최된 AACR 2022에서 소개된 10개의 New Drug Candidate 화합물
<원문보기>
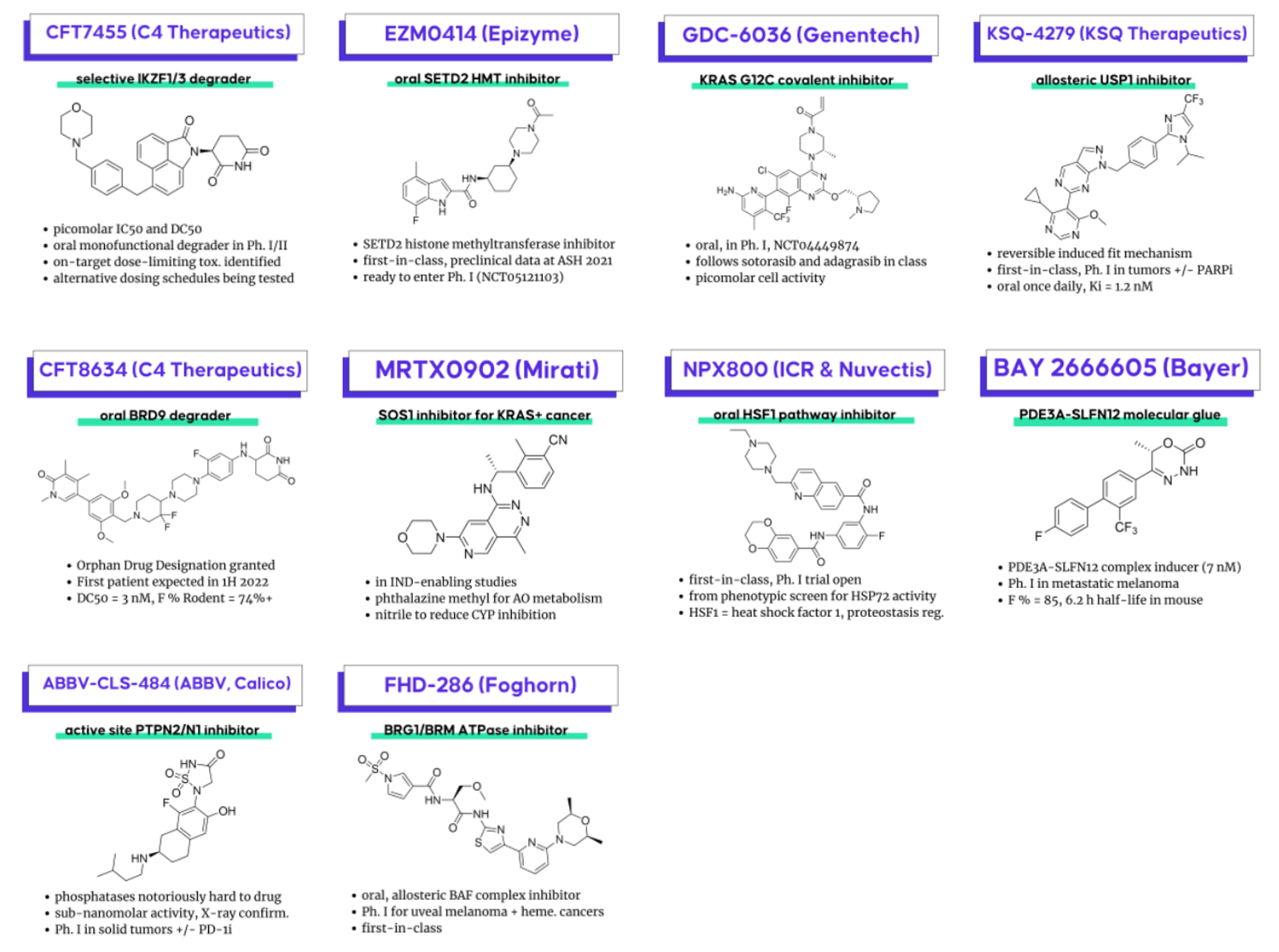
CFT7455 (C4 Therapeutics) – a selective IKZF1/3 degrader for relapsed/refractory multiple myeloma (Ph. I/II – NCT04756726) (one of two degraders from C4 disclosed in these sessions). C4 also recently disclosed clinical data from this molecule, where they identified on-target dose-limiting toxicity, and trying to increase the therapeutic index with an alternative dosing schedule. It will be interesting to watch this as a case study for the degrader mechanism of action as alternative dosing is challenging to pull off even with non-degrader modalities.
FHD-286 (Foghorn Therapeutics) – a first-in-class, oral, allosteric BAF chromatin remodeling complex inhibitor (via BRG1 and BRM ATPase inhibition) in two Ph. I trials for metastatic uveal melanoma (NCT04879017) and advanced hematologic malignancies (NCT04891757)
GDC-6036 (Genentech) – an oral, covalent mutant KRAS G12C inhibitor in Ph. I (NCT04449874), following adagrasib and sotorasib in the class
EZM0414 (Epizyme) – a first-in-class, oral, selective inhibitor of SETD2, a histone methyltransferase ready to enter Ph. I (NCT05121103) – not yet recruiting. Preclinical data had previously been prevented at ASH 2021. Interestingly the lead scaffold is isomeric to the most recent SETD2 compound patent from Epizyme (WO2021168313)
CFT8634 (C4 Therapeutics) – received Orphan Drug Designation from the FDA, first patient in expected this year
BAY 2666605 (Bayer) – a PDE3A-SLFN12 molecular glue (“velcrin”), induces a complex with a crystal structure published in collaboration with the Broad Institute. Nice progress from a starting point (DNMDP) shared by Broad scientists and developed in collaboration with Bayer. Hope it does well in its Ph. I trial in metastatic melanoma and other solid tumors NCT04809805.
KSQ-4279 (KSQ Therapeutics, Inc.) – an allosteric, first-in-class USP1 inhibitor going into Ph. I in tumors alone and in combo with PARP inhibition. Has a reversible induced fit mechanism
ABBV-CLS-484 (AbbVie, Calico Life Sciences, Broad Institute of MIT and Harvard) – an X-ray confirmed, picomolar active-site inhibitor of phosphatase PTPN2/N1 – phosphatases typically thought of as undruggable. Going into Ph. I in combo with checkpoint inhibition. Phosphatase inhibitors has always been polar, but they managed to get this one just right!
MRTX0902 (Mirati Therapeutics) – a SOS1 inhibitor for KRAS+ cancer following Boehringer Ingelheim’s BI 1701963 – BI and Mirati previously collaborated on BI’s SOS1 inhibitor with MRTX849
NPX800 – an oral heat shock factor 1 (HSF1) pathway inhibitor with a Ph. I trial open (first-in-class); from a phenotypic screen for HSP72 activity
<원문보기>
Reviewed by Dr. Julien Lefranc, a Principal Scientist at Merck KGaA in Darmstadt and a member of the Early Career Advisory Board at ChemMedChem.

<원문보기>
CFT7455 (C4 Therapeutics) – a selective IKZF1/3 degrader for relapsed/refractory multiple myeloma (Ph. I/II – NCT04756726) (one of two degraders from C4 disclosed in these sessions). C4 also recently disclosed clinical data from this molecule, where they identified on-target dose-limiting toxicity, and trying to increase the therapeutic index with an alternative dosing schedule. It will be interesting to watch this as a case study for the degrader mechanism of action as alternative dosing is challenging to pull off even with non-degrader modalities.
FHD-286 (Foghorn Therapeutics) – a first-in-class, oral, allosteric BAF chromatin remodeling complex inhibitor (via BRG1 and BRM ATPase inhibition) in two Ph. I trials for metastatic uveal melanoma (NCT04879017) and advanced hematologic malignancies (NCT04891757)
GDC-6036 (Genentech) – an oral, covalent mutant KRAS G12C inhibitor in Ph. I (NCT04449874), following adagrasib and sotorasib in the class
EZM0414 (Epizyme) – a first-in-class, oral, selective inhibitor of SETD2, a histone methyltransferase ready to enter Ph. I (NCT05121103) – not yet recruiting. Preclinical data had previously been prevented at ASH 2021. Interestingly the lead scaffold is isomeric to the most recent SETD2 compound patent from Epizyme (WO2021168313)
CFT8634 (C4 Therapeutics) – received Orphan Drug Designation from the FDA, first patient in expected this year
BAY 2666605 (Bayer) – a PDE3A-SLFN12 molecular glue (“velcrin”), induces a complex with a crystal structure published in collaboration with the Broad Institute. Nice progress from a starting point (DNMDP) shared by Broad scientists and developed in collaboration with Bayer. Hope it does well in its Ph. I trial in metastatic melanoma and other solid tumors NCT04809805.
KSQ-4279 (KSQ Therapeutics, Inc.) – an allosteric, first-in-class USP1 inhibitor going into Ph. I in tumors alone and in combo with PARP inhibition. Has a reversible induced fit mechanism
ABBV-CLS-484 (AbbVie, Calico Life Sciences, Broad Institute of MIT and Harvard) – an X-ray confirmed, picomolar active-site inhibitor of phosphatase PTPN2/N1 – phosphatases typically thought of as undruggable. Going into Ph. I in combo with checkpoint inhibition. Phosphatase inhibitors has always been polar, but they managed to get this one just right!
MRTX0902 (Mirati Therapeutics) – a SOS1 inhibitor for KRAS+ cancer following Boehringer Ingelheim’s BI 1701963 – BI and Mirati previously collaborated on BI’s SOS1 inhibitor with MRTX849
NPX800 – an oral heat shock factor 1 (HSF1) pathway inhibitor with a Ph. I trial open (first-in-class); from a phenotypic screen for HSP72 activity
<원문보기>
Reviewed by Dr. Julien Lefranc, a Principal Scientist at Merck KGaA in Darmstadt and a member of the Early Career Advisory Board at ChemMedChem.